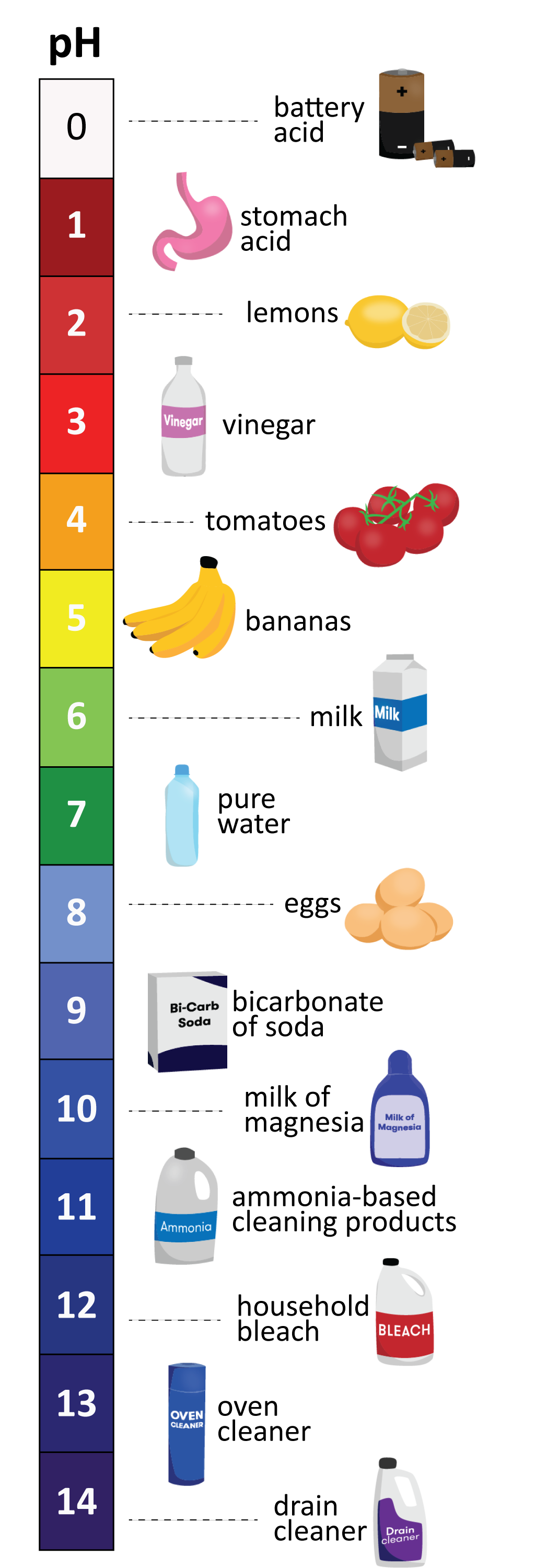
Acidity is a measure of the concentration of hydrogen ions (H⁺) present when a substance is dissolved in a liquid. It is measured on the pH scale, which stands for 'potential of Hydrogen ions'. The pH scale generally ranges from 1–14, one being the most acidic. There are two things that contribute to an acid’s pH value: the acid’s strength, and its concentration. The strength of an acid is determined by its readiness to give up its hydrogen ions (or its ability to split up water molecules to produce hydrogen ions)—the more hydrogen ions it releases when dissolved in water, the stronger an acid it is, and the lower its pH will be. But we can also get a lot of hydrogen ions in a solution if we simply have a high concentration of the acid—there’s more of it, so more hydrogen ions.
Pure water has a pH of 7, and is considered neutral. Values below 7 are acidic, and values above 7 are called basic, or alkaline. Most drinking water usually sits between 6.5 and 7.5. Vinegar has a pH of 3, a cola drink around 2.5 and battery acid 1 (very acidic). On the other end of the scale, heavy duty cleaning products can have pH of 12–14 (very alkaline). The pH scale is logarithmic, which means that each value is ten times greater or less than those on either side of it. So, a solution with a pH of 4 has 10 times more hydrogen ions in it than a solution with a pH of 5, and 100 times more than a pH of 6.
Oh, and a lemon has a pH of around 2. It’s pretty acidic—and that’s why it’s sour.

###some pull quote here### ##some citation here with a #link# --> This article was adapted from Academy website content reviewed by the following experts: Professor Ove Hoegh-Guldberg FAA Director, Global Change Institute and Professor, Marine Science, University of Queensland; Prof Emma Johnston Dean of Science and Head of the Applied Marine and Estuarine Ecology Lab, University of New South Wales; Dr Richard Matear Chief Research Scientist, Ocean and Atmosphere, CSIRO